Confocal and Multiphoton Microscopy Core
The goal of the Confocal and Multiphoton Microscopy Core is to support the research programs of a diverse group of NIH funded users and other users located in the Schools of Dentistry, Pharmacy, Medicine and Chemical and Biological Sciences at the University of Missouri-Kansas City. The core supports members of the UMKC Center of Excellence in the Study of Dental and Musculoskeletal Tissues (CEMT), as well as the research community at UMKC at large. The confocal microscopy core resource is also available to the wider academic and commercial communities in the Kansas City Region. The core can accommodate a wide range of traditional high-resolution microscopy and spectral imaging projects as well as supporting biophysical methods and applications development. Image analysis automation and high content microscopy image analysis services are also available.
Microscopes at a Glance:
- Leica inverted SP8 Stellaris Confocal Microscope
- Live Cell Imaging (Incubation Chamber with CO2, Temperature and Humidity Control)
- Multi-Spectral Confocal Imaging of Fixed Cells and Tissue Samples
- Tiled image capture for High Resolution Imaging of Large Areas
- Optical Sectioning and 3-D Image Capture (also in Time-lapse)
- Spectral Profiling
- Förster Resonance Energy Transfer (FRET)
- Fluorescence Recovery After Photobleaching (FRAP)
- Fluorescence Loss in Photobleaching (FLIP)
- Fluorescence Lifetimes Imaging Microscopy (FLIM)
- High Speed Imaging for Live Cell Kinetics
- Multi-point time-lapse movie collection.
- Physiological indicators imaging (for example, calcium imaging and pH imaging)
- Real-Time Optical Deconvolution ( > 120 nm FWHM)
- Leica upright SP8 Multiphoton and Confocal Microscope
- Multi-Photon Imaging and Second Harmonic Generation Imaging.
- Intravital Imaging
- Live Cells and Fixed Tissue Imaging
- Förster Resonance Energy Transfer (FRET)
- Fluorescence Recovery After Photobleaching (FRAP)
- Fluorescence Loss in Photobleaching (FLIP)
- High Speed Imaging for Live Cell Kinetics
- Multi-point time-lapse movie collection.
- Physiological indicators imaging (for example, calcium imaging and pH imaging)
- Keyence BZ-X810 Epifluorescence High Content Imaging System
- Bright-Field Imaging with Color Camera for Tissue Histology
- Widefield Multi-Color Fluorescence Imaging
- Image Tiling
- High-Content Imaging Cytometry Analysis
Links and Downloads
- ImageJ
- Fiji (Fiji is Just ImageJ)
- LAS X Office (Leica offline Image processing software.)
- CellProfiler
- CellProfiler Analyst
- Imaris
- OMERO
- KNIME

Specifications at a glance
- 5X, 10X, 20X, 40X, 60X and 100X objectives.
- Max Resolution ~125 nm FWHM.
- Excitation Laser wavelengths ranging from 405 nm (Diode), 440nm-790nm (White Light Laser).
- Conventional and Resonance Scanners for scan speeds ranging from 100 Hz-8000 Hz.
- Leica’s Hybrid Detectors combining the best of Photo Multiplier Tubes and Avalanche Photodiode Detectors. Single photon counting mode available for quantitative fluorescence detection.
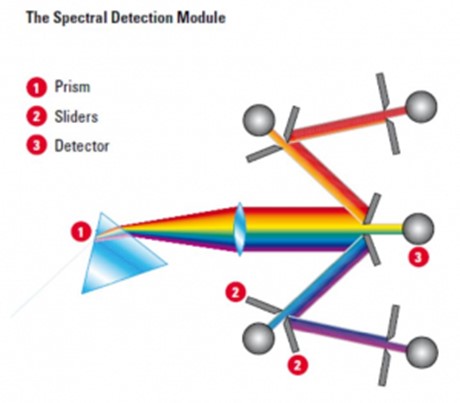
- Acousto-Optical Tunable Filter and Acousto-Optical Beam Splitter combined with prism and sliders permit precise choice of excitation and emission wavelengths from 420nm-800nm with customizable detector bandwidths.
- 5 Hybrid Detectors for up to 8 simultaneously recorded channels.
- 1 PMT for Transmission Images (Producing images similar to wide field DIC ).
- High Speed Pulsed White Light Laser permits time-gated single photon counting and photon arrival time measurements (Fluorescent Lifetimes Measurement)
- Galvo-Z Stage for fine control of Z – position and Z-axis scanning.
- Applications include: Single photon counting, spectral profiling and spectral deconvolution, 2-D and 3-D imaging, large area image tiling, 2D and 3D co-localization, Förster Resonance Energy Transfer (FRET), Fluorescence Recovery After Photobleaching (FRAP), Fluorescence loss after photobleaching (FLIP), Fluorescence Lifetime Imaging Microscopy (FLIM), on-the-fly optical deconvolution, programmable time-lapse imaging including 2-D and 3D multi-point time-lapse imaging, monitoring of cells under physiological imaging conditions (Temp, CO2, and humidity control) and monitoring of genetically-encoded indicators of physiology and small molecule physiological indicators.
Objectives
| Objective | Immersion Type |
NA | Pixel Resolution (green) | Working Distance |
| 2.5x Fluorite Plan | Air | .07 | 2 µm | 11.2 mm |
| 5x Plan Fluorite | Air | 0.15 | 920 nm | 14.0 mmm |
| 10x Plan Apochromat | Air | 0.4 | 350 nm | 2.2 mm |
| 20x Plan Apochromat | Air | 0.7 | 200 nm | 0.59 mm |
| 20x Plan Apochromat | Multi | 0.75 | 185 nm | 0.67 mm |
| 25x Plan Apochromat | Water | 0.95 | 150 nm | 2.5 mm |
| 40x Plan Apochromat | Oil | 1.25 | 110 nm | 0.24 mm |
| 63x Plan Apochromat | Oil | 1.4 | 100nm | 0.14 mm |
| 100x Plan Apochromat | Oil | 1.44 | 95 nm | 0.10 mm |
Lasers
| Laser | Wavelengths (nm) |
Maximum Power (mW) |
| UV diode | 405nm | 50 mW |
| White Light Laser | 440-790nm | 2.5 W (peak power, pulsed laser), 20 mW average. |
Scanners
The availability of both a conventional confocal scanner and a resonance scanner allows the user to optimize images for both resolution and speed.
| Scanners | Frame Scan Speed |
Line Scan Speed |
Frame Rate
|
Max Image Size |
Beam Park |
Spot Collection Rate |
| Conventional | 10-2600 Hz | 100-2000 Hz | .019/sec-4.94/sec | 9600x 9600 | no | N/A |
| Resonance | 8000 Hz | 16000 Hz | 3.84/sec-142.86/sec | 2048x 2048 | no | N/A |
Excitation
Acousto-Optical Tunable Filter (AOTF): An Acousto-Optical Optical Tunable Filter is a high transmission efficiency, beam splitting crystal that both tunes the excitation wave-length of the lasers used for fluorophore excitation and that transmits the emission light for subsequent detection. In this case, a sound wave is directed at the crystal which rapidly alters the diffraction angle of the lasers entering the prism. This alters the excitation wavelength reaching the sample. The use of an acousto-optical tunable filter in place of a traditional beam splitter allows for high speed, programmable excitation light switching and high-transmission efficiency of collected light.
Detection
The Leica SP5 employs a prism and tunable sliders to separate the collected light into channels for detection at Leica’s hybrid detectors (HyD). Leica’s Hybrid detectors are a newer type of detector technology that combines the benefits of both photomultiplier tubes (PMTs) and avalanche photodiode (APD) detectors while avoiding some of their limitations. Compared to PMTs, Hybrid detectors offer several advantages such as higher quantum efficiency, faster time response, and better linearity. They can detect single photons with high efficiency and have a larger dynamic range than PMTs. Additionally, Hybrid detectors have low noise levels and are less prone to saturation, which can be an issue with PMTs. Compared to APD detectors, Hybrid detectors have a lower excess noise factor, which means they can achieve higher signal-to-noise ratios for low light level detection. They are also less sensitive to temperature changes and can operate at higher temperatures than APDs. These detectors (Leica’s HyD detectors allow for low light level imaging with very little dark noise.) Sliding filter notches permit users to tune their spectral collection window without the need for specific dichroic filters for each dye (below). The Leica SP5 employs a prism and tunable sliders to separate the collected light into channels for detection at the Hybrid Detectors. This allows users to specify each spectral collection window.
Detectors
| Detector | Number Available |
Peak Quantum Efficiency |
Contrast (A.U.) (500 nm) |
Bit Depth | Photon Counting Mode |
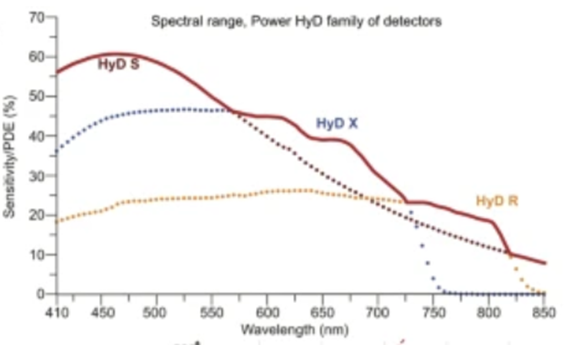
| HyD-S | 4 | 60% | 5 | 8, 12, 16 | Yes |
| HyD-X | 1 | 45% | 160 | 8, 12,16 | Yes |
Automated Stage
Our SP8 Stellaris system features a fully automated stage for movement in all three dimensions. X-Y tiling and mark and find features in the software can be automated to minimize the time spent with the microscope during image acquisition and to allow repeated imaging of many regions at timed intervals. This permits time lapse experiments that can be performed simultaneously at multiple points. The Galvo-Z stage allows for fine z-plane adjustment necessary to perform 3-D reconstructions of samples within the focal resolution of the microscope. Adaptive Focus Control and Best Focus features in the software allow for consistent maintenance of the z-position of the stage over long time scales.
Imaging Applications
Live Cell Imaging: Our confocal microscope is contained within a temperature controlled Cage Incubator from Okolabs. A stage-top incubator unit from Tokai Hit CO2 permits fine temperature control, humidity regulation, and CO2 maintenance during imaging.
Analog based Intensity Imaging and single photon counting . Analog based Intensity Imaging and single photon counting are available for all fluorophores, including those that emit into the far red (<800nm). Emission filters and bandwidths are not a concern with the Prism and Slider system of the Leica SP5. With proper controls, single photon counting permits more quantitative imaging.
Spectral Profiling. Spectral profiling is a technique used to separate and quantify the emission spectra of multiple fluorescent signals within a sample. In spectral profiling the microscope sequentially acquires multiple images at different wavelengths across a range of excitation and emission wavelengths. Spectral profiling can be used to identify the excitation and emission spectra of different fluorescent signals in a sample, and can be used to distinguish between signals with similar spectral properties. This technique is particularly useful when working with complex samples with multiple fluorescent species or when trying to differentiate between autofluorescence and fluorescent signals.
Linear Unmixing. Linear unmixing can be used in the context of a confocal microscope to separate signals from multiple fluorescent labels that may overlap in their emission spectra. This is because the fluorescence signal detected by the confocal microscope is a linear combination of the emissions from the different labels used to stain the sample. By using linear unmixing, it is possible to estimate the contribution of each label to the overall fluorescence signal in each pixel of the image. This allows researchers to visualize the spatial distribution of each label separately, providing a clearer and more accurate representation of the sample.
2D and 3-D imaging. A confocal microscope is capable of acquiring images in both 2D and 3D with an automated stage. In 2D imaging, the microscope captures a single optical section of the sample at a specific depth using a laser beam and a pinhole aperture. This allows for high-resolution imaging of a thin slice of the sample, which can be useful for visualizing cellular structures and interactions. In 3D imaging, the microscope captures multiple optical sections at different depths within the sample, which are then reconstructed into a 3D image. This provides a more complete view of the sample, allowing researchers to analyze the spatial relationships between different structures and obtain a better understanding of the 3D architecture of the tissue.
2D and 3D Tiling: Tiling on a Stellaris microscope involves acquiring and stitching together multiple images to create a single, larger image of a sample. In 2D tiling, the microscope captures multiple images of adjacent regions of a sample at high resolution, which are then automatically stitched together using software to create a larger, high-resolution image of the entire sample. This is particularly useful for imaging large tissue sections, where a single field of view may not be sufficient to capture all the relevant information. In 3D tiling, the microscope captures multiple images of the same region of a sample at different focal planes, which are then reconstructed into a 3D image volume. This allows researchers to capture a 3D image of a large sample with high resolution and detail, enabling them to analyze the spatial relationships between different structures and obtain a better understanding of the 3D architecture of the tissue samples.
Co-localization studies. Co-localization studies are used to determine the extent of overlap between two or more fluorescently labeled molecules within a cell or tissue. Analysis is limited only by the resolution of the microscope (~120nm FWHM) and the spectral overlap between fluorophores. Up to 5 channels in a single scan are possible. Multiple separate scans may be used to minimize spectral bleed-through. Quantitative Co-localization in 2-D and 3D is provided through FIJI/ImageJ.
Förster Resonance Energy Transfer (FRET) Studies. Software support for Sensitized Emission FRET and Photobleaching FRET is available. In Sensitized emission FRET, the donor fluorophore is excited, and the energy is transferred to the acceptor fluorophore which then emits light at a longer wavelength. In photobleaching FRET the acceptor fluorophore is photobleached, unquenching donor fluorescence. In both cases, FRET efficiencies are calculated and can be used to measure the distance between the donor and acceptor fluorophores (within about 0-12 nm).
Fluorescence Recovery after Photobleaching. (FRAP): Software support for FRAP is available. FRAP is a microscopy technique used to study the movement of fluorescently labeled molecules within living cells. In this technique, a specific region of the cell is bleached with a high-intensity laser, which destroys the fluorescence of the molecules within that region. The recovery of fluorescence in the bleached region is then monitored over time as fluorescent molecules from the surrounding unbleached regions diffuse into the bleached region. The rate of fluorescence recovery is affected by the mobility and concentration of the fluorescently labeled molecules and can provide information about the dynamics of protein-protein interactions, diffusion rates, and membrane trafficking. The technique is commonly used to study the movement and turnover of proteins, lipids, and other molecules within living cells. Fluorescence Loss in Photobleaching (FLIP) is also supported in this application.
Fluorescence Lifetime Imaging (FLIM) Fluorescence lifetime imaging is a microscopy technique that measures the decay of fluorescence over time. Unlike traditional fluorescence microscopy, which measures only the intensity of fluorescence, FLIM provides information about the lifetime of the fluorescent signal, which is dependent on the microenvironment and molecular interactions of the fluorescent molecule. In FLIM, a pulsed laser is used to excite a fluorescent molecule, and the resulting emission is measured over time. The fluorescence decay curve is analyzed to determine the fluorescence lifetime, which is the average time that the molecule spends in the excited state before returning to the ground state. FLIM can be used to study molecular interactions, protein-protein interactions, and other biochemical processes in living cells. For example, FLIM-FRET (Fluorescence Resonance Energy Transfer) can be used to measure the distance between fluorescently labeled molecules and thus provide information about their interactions.
On-the-fly Optical Deconvolution. Leica’s “Lightning” optical deconvolution software is an advanced image processing tool used to improve the resolution and quality of images acquired using fluorescence microscopy. The software uses a deconvolution algorithm to correct for the effects of optical distortion and scattering that occur when light passes through a biological sample, resulting in images that are sharper, clearer, and more detailed. The deconvolution algorithm uses a mathematical model to reconstruct the original image from the blurred image acquired by the microscope. The “Lightning” software uses a machine learning approach to speed up the deconvolution process, allowing on-the-fly visualization, which can otherwise be time-consuming and computationally intensive.
Multipoint Time-lapse Imaging: Multipoint time-lapse imaging is performed by automatically acquiring images of multiple distinct regions of interest over time. This technique is useful for increasing sample sizes while studying dynamic cellular processes, such as cell migration, cell division, and protein trafficking in living cells or tissues.
Imaging of Physiological Indicators: A range of detectors and filters enable the imaging of physiological indicators, such as those for calcium, pH and a variety of other molecular signaling pathways. These indicators can be visualized using fluorescent dyes, which change their fluorescence properties in response to changes in physiological conditions. The Stellaris microscope can image the indicators in real-time, providing insights into cellular function and organization of signaling pathways.
Programmable Time-lapse imaging: The programming module in LASX provides a user-friendly interface for controlling image capture in time-lapse experiments on the Leica Stellaris microscope. Users can program the system to automatically acquire images at specific time intervals or at differing specific time intervals all within a single recording. This can be repeated at multiple locations within a single sample. The programming module allows users to create customized imaging protocols tailored to their specific experimental needs, streamlining the time-lapse imaging process and reducing the need for manual intervention over pro-longed imaging periods (even up to 4-5 days.)

Specifications at a glance
- 16x, 25x, 40x, and 63x IR objectives
- Multiphoton Excitation Laser wavelengths of 690-1040nm.
- Simultaneous two channel detection using filter cubes for DAPI/FITC, FITC/TRITC or CFP/YFP.
- Non-descanned Two-channel Detection by two hybrid PMT/APD detectors.
- Single photon excitation wavelengths of 488nm and 552nm.
- Descanned, confocal detection using 1 standard PMT for confocal detection.
- Sequential spectral detection using a prism and slider separation for emission wavelengths from 400nm-800nm with customizable bandwidths.
- Transmission image detection using one standard PMT.
- Conventional and Resonance Scanners for line scan speeds ranging from 400hz-1800Hz (conventional scanner) and 8000 Hz (resonance scanner).
- Applications include: Two color, temperature controlled, Intravital Imaging in multi-photon mode, Forward Scatter Second Harmonic Generation Imaging and Back Scatter Second Harmonic Generation imaging, temperature controlled live-cell imaging, image tiling in 2D and 3D, and multi-point live cell time-lapse Imaging, intensity based imaging of up to 5 sequential channels in single photon mode, spectral imaging, FRET, FRAP, and FLIP
Objectives
| Objective | Immersion Type |
NA | Pixel Resolution (green) | Working Distance |
| 10x HC IR Apochromat Dipping | Water | 0.3 | 480 nm | 3.6mm |
| 16x Fluortar Dipping | Multi | 0.6 | 230 nm | 2.5mm |
| 25x Plan IR Apochromat | Water | .95 | 150 nm | 2.5 mm |
| 40x Plan IR Apochromat | water | 1.10 | 130 nm | 0.65 mm |
| 63x Plan Apochromat Dipping | water | 0.90 | 160 nm | 2.2 mm |
Lasers
| Laser | Wavelengths | Maximum Power |
| 488 nm solid state | 488 | 20 mW |
| 552 nm solid state | 552 | 20 mW |
| MaiTai HP DeepSee | 690-1040 | 1.5 W (average at 800nm)
|
Scanners
The availability of both a conventional confocal scanner and a resonance scanner allows the user to optimize for resolution or speed.
| Scanner | Line Scan Speed |
Frame Rate (512×512) |
FOV | Max Image Size |
Beam Park |
Spot Collection Rate |
| Conventional | 100-2000 Hz | 2/sec | 22 mm- 3 mm | 9600x 9600 | yes | 40 MHz |
| Resonance | 8,000 Hz | 25/sec-200/sec | 15 mm | 1024x 1024 | no | N/A |
Non-Descanned Detection
For high sensitivity multiphoton signal acquisition, non-descanned detectors are used in conjunction with filter cubes for maximal light gathering with minimal light dispersion. Current filter cube configurations are listed in the table.
| Cube | Dichroic (Beam Splitter) | Filter 1 | Filter 2 |
| DAPI/FITC | RSP 495 | 460/50 | 525/50 |
| FITC/TRITC | RSP 560 | 525/50 | 585/40 |
| CFP/YFP | RSP505 | 483/32 | 535/30 |
Descanned Detection
AOBS: The use of an Acousto-Optical Beam Splitter in place of a traditional beam splitter allows for programmable, high-transmission direction of collected light.
Detectors
| Detector | Number Available |
Quantum Efficiency (500nm) |
Contrast (A.U.) (500 nm) |
Bright R | Bit Depth | Photon Counting Mode |
| Standard (descanned) | 1 | 25% | 5 | No | 12-16 | No |
| HyD (Non-descanned) | 2 | 45% | 160 | Yes | 12 | Yes |
Imaging Applications
Multiphoton based illumination. Multiphoton based imaging is a type of technique that uses longer-wavelength light to excite fluorescent molecules within a sample. Unlike traditional fluorescence microscopy, where a single photon is absorbed by a fluorophore to produce fluorescence, multiphoton imaging uses two or more photons of lower energy, which are absorbed simultaneously to excite the fluorophore. The photons are delivered to the sample via a pulsed laser, typically a titanium-sapphire laser, which produces ultra-short pulses of light with high peak power. The light is then focused through a microscope objective lens onto the sample, which is typically mounted on a movable stage for precise positioning. The two-photon excitation process occurs only within a confined volume around the focal point, where the density of photons is highest. This results in very low photobleaching and phototoxicity, above and below the plane of focus. This makes multiphoton imaging ideal for live-cell imaging and deep-tissue imaging.
Intravital Imaging. Superior signal penetration of the long wavelengths used in multiphoton microscopy make the SP8 multiphoton ideal for intravital imaging with the proper specimen holder/ stereotaxic device and an IACUC approved protocol.
Second Harmonic Generation (SHG) imaging. Second Harmonic Generation (SHG) imaging is a nonlinear optical imaging technique that uses two photons of the same frequency to generate a new photon with twice the energy and half the wavelength. This phenomenon occurs in materials that lack inversion symmetry, such as collagen fibers in biological tissue. When a sample is illuminated with a high-intensity laser beam, the collagen fibers generate a second harmonic signal that is detected and used to produce an image. This allows for label-free imaging of biological structures, such as collagen fibers in skin, tendons, and bone, with high spatial resolution and contrast. Forward scatter SHG detection and backscatter SHG detection are available on the SP8 multiphoton microscope.
Analog based Intensity Imaging. In single photon mode Analog based intensity imaging is available for all fluorophores with emissions ranging from 400nm to 800nm. Emission filtering and detection bandwidths are flexible with the prism and slider system of the Leica SP8.
Spectral Profiling. Spectral profiling is a technique used to separate and quantify the emission spectra of multiple fluorescent signals within a sample. In spectral profiling the microscope sequentially acquires multiple images at different wavelengths across a range of excitation and emission wavelengths. Spectral profiling can be used to identify the excitation and emission spectra of different fluorescent signals in a sample, and can be used to distinguish between signals with similar spectral properties. This technique is particularly useful when working with complex samples with multiple fluorescent species or when trying to differentiate between autofluorescence and fluorescent signals.
2D and 3-D imaging. A multiphoton microscope can acquire images in both 2D and 3D while exciting a very small focal volume. In 2D imaging, the microscope captures a single optical section of the sample at a specific depth using a pulsed IR laser beam. A pinhole aperture is available for detection. This allows for high-resolution imaging of a thin optical slice of the sample, which can be useful for visualizing cellular structures and interactions. In 3D imaging, the microscope captures multiple optical sections at different depths within the sample, which are then reconstructed into a 3D image. This provides a more complete view of the sample, allowing researchers to analyze the spatial relationships between different structures and obtain a better understanding of the 3D architecture of the tissue.
2D and 3D Tiling: Tiling on an SP8 Multiphoton microscope involves acquiring and stitching together multiple images using and automated stage to create a single, larger image of a sample. In 2D tiling, the microscope captures multiple images of adjacent regions of a sample at high resolution, which are then automatically stitched together using software to create a larger, high-resolution image of the entire sample. This is particularly useful for imaging large tissue sections, where a single field of view may not be sufficient to capture all the relevant information. In 3D tiling, the microscope captures multiple images of the same region of a sample at different focal planes, which are then reconstructed into a 3D image volume. This allows researchers to capture a 3D image of a large sample with high resolution and detail, enabling them to analyze the spatial relationships between different structures and obtain a better understanding of the 3D architecture of the tissue samples.
Co-localization studies. Co-localization studies are used to determine the extent of overlap between two or more fluorescently labeled molecules within a cell or tissue. Analysis is limited only by the resolution of the microscope and the spectral overlap between fluorophores. Up to 5 channels in sequential scans are possible. Multiple separate scans may be used to minimize spectral bleed-through. Quantitative Co-localization in 2-D and 3D is provided through FIJI/ImageJ.
Förster Resonance Energy Transfer (FRET) Studies. Software support for Photobleaching FRET is available. In photobleaching FRET the acceptor fluorophore is photobleached, unquenching donor fluorescence. FRET efficiencies are calculated within the software and can be used to measure the distance between the donor and acceptor fluorophores (within about 0-12 nm).
Fluorescence Recovery after Photobleaching. (FRAP): Software support for FRAP is available. FRAP is a microscopy technique used to study the movement of fluorescently labeled molecules within living cells. In this technique, a specific region of the cell is bleached with a high-intensity laser, which destroys the fluorescence of the molecules within that region. The recovery of fluorescence in the bleached region is then monitored over time as fluorescent molecules from the surrounding unbleached regions diffuse into the bleached region. The rate of fluorescence recovery is affected by the mobility and concentration of the fluorescently labeled molecules and can provide information about the dynamics of protein-protein interactions, diffusion rates, and membrane trafficking. The technique is commonly used to study the movement and turnover of proteins, lipids, and other molecules within living cells. Fluorescence Loss in Photobleaching (FLIP) is also supported in this application.
Optical Deconvolution. Optical deconvolution software is an advanced image processing tool used to improve the resolution and quality of images acquired using fluorescence microscopy. The software uses a deconvolution algorithm to correct for the effects of optical distortion and scattering that occur when light passes through a biological sample, resulting in images that are sharper, clearer, and more detailed. The deconvolution algorithm uses a mathematical model to reconstruct the original image from the blurred image acquired by the microscope. Deconvolution of multiphoton based images can be performed with FIJI/ImageJ.
Multipoint Time-lapse Imaging: Multipoint time-lapse imaging is performed by automatically acquiring images of multiple distinct regions of interest over time. This technique is useful for increasing sample sizes while studying dynamic cellular processes, such as cell migration, cell division, and protein trafficking in living cells or tissues.
Imaging of Physiological Indicators: A range of detectors and filters enable the imaging of physiological indicators, such as those for calcium, pH and a variety of other molecular signaling pathways. These indicators can be visualized using fluorescent dyes, which change their fluorescence properties in response to changes in physiological conditions. The Stellaris microscope can image these indicators in real-time, providing insights into cellular function and signaling pathways.
Live Cell Imaging: Our multiphoton microscope is contained within a temperature controlled chamber. Temperature and recording conditions may be preserved over pro-longed imaging periods.
Stereotaxic equipment. Stereo-tactic equipment and anesthesia administration is now available for mice within the multiphoton microscope setup, allowing for precise and controlled targeting of brain/skull regions during imaging experiments. This technology offers researchers a powerful tool for investigating neural circuitry and behavior in vivo with high spatial resolution.

Specifications at a glance
- 4X, 10X, 10X Phase, 20X, 20X Phase, 40X objectives
- Automated high-throughput imaging and analysis of biological samples
- Brightfield (Color Camera), phase-contrast (Grayscale Camera), and fluorescence (Grayscale Camera) imaging modalities
- Multi-channel fluorescence imaging
- Multiwell plate and slide compatibility
- User-friendly software interface for ease of use and data analysis.
- Compatible with a wide range of fluorescent dyes and probes
- Motorized stage and autofocus capabilities for large-area tiled imaging
- Time-lapse imaging for dynamic studies
- Advanced image analysis software for automated cell counting, morphological analysis, and tracking of cell behavior over time.
Imaging Applications
Automated and semi-automated microscope. The Keyence BZ-X810 Imaging System is an advanced, automated microscope that is used for high-throughput imaging and analysis of biological samples. This microscope system offers a range of imaging modalities, including brightfield, phase-contrast, and fluorescence, making it suitable for a wide range of applications. One of the key features of the BZ-X810 is its ability to perform high-throughput imaging and analysis. The system is equipped with a motorized stage and autofocus capabilities, which allow for rapid and precise positioning of the sample. This, in turn, enables the user to image multiple samples in a short period of time. The system can accommodate a variety of sample formats, including multiwell plates, slides, and other types of microscopes slides. The system also has a user-friendly software interface that enables easy control and manipulation of the images.
Four channels. The BZ-X810 is also compatible with a wide range of fluorescent dyes and probes, making it suitable for a variety of research applications. The system is equipped with a range of excitation and emission filters, allowing the user to select the optimal wavelength for their application. Four channels are available.
High Resolution with minimized Phototoxicity and Photobleaching. The BZ-X810 also offers high-resolution imaging with some protections against phototoxicity and photobleaching. This is particularly important for imaging living cells, as phototoxicity and photobleaching can damage or destroy the cells over time. The system uses a range of advanced optics and sensors to minimize the impact of these factors, allowing for long-term imaging of living cells. The system can also perform time-lapse imaging, allowing the user to monitor changes in a sample over time. This is particularly useful for studying dynamic processes such as cell migration, proliferation, and differentiation.
High Content Analysis. The BZ-X810 comes equipped with advanced image analysis software that allows for automated cell counting, morphological analysis, and tracking of cell behavior over time. This software enables the user to process large amounts of data quickly and accurately, facilitating high-throughput analysis. Alternately, High Content Imaging and Analysis can also be performed using The CellProfiler/CellProfiler Analyst Software platform.
Registration and Training of Users:
- Contact Dr. David Moore for a consultation to determine whether assisted operation, independent operation or advanced user operation is best suited to your experimental needs.
- Please register on the confocal and multiphoton microscopy core online reservation system at: https://umkcconfocal.bookedscheduler.com
- Once registered, you may schedule a training session at your convenience. Please remember to reserve time both on the instrument and with a staff member if it is your first time using a particular instrument.
- Training may only be performed by one of the Core Co-Directors; Dr. Moore, or Dr. Dallas.
- Assisted Use: Initial training sessions will permit use of the instruments Monday-Friday 8am-5pm and will be billed as assisted imaging. A co-director will be present for help during these sessions.
- Independent use of the instrumentation requires an additional training session where it is preferable that a Principal Investigator or Senior Advisor to the user attend the training to verify that the user’s expertise is sufficient for independent use of the instrument. Advisor sign-off is required. It is not necessary that a co-director be present following this training.
- Advanced user status, generally, will require users to log a requisite number of required hours and demonstrate a higher level of competency. Advanced user operation will be at the discretion of the Core Co-Directors.
Hourly Fees for Usage of the Leica SP8 Stellaris Confocal Microscope
| User Category | UMKC | Academic (non-UMKC) | Commercial |
| Assisted | $40.00 | $50.00 | $120.00 |
| Independent | $25.00 | $35.00 | $85.00 |
| Time series*/Off peak** | $15.00 | $20.00 | n/a |
| Fee-for-Service | Inquire | $95.00 | $170.00 |
* Time series extending more than 8 hours ($500 cap for long term, 3-4 day time-lapse experiments)
**Off Peak hours will be defined as ending at 10 am and beginning after 4 pm. You must be an advanced user to use instruments in off peak hours. (This kind of use requires that you get building access and room access well in advance of intended off peak use.)
Hourly Fees for Usage of the Leica SP8 Multiphoton Microscope
| User Category | UMKC | Academic (non-UMKC) | Commercial |
| Assisted | $55.00 | $65.00 | $180.00 |
| Independent | $35.00 | $45.00 | $110.00 |
| Time series*/Off peak** | $25.00 | $30.00 | n/a |
| Fee-for-Service | Inquire | $120.00 | $220.00 |
* Time series extending more than 8 hours ($600 cap for long term time-lapse experiments)
**Off Peak hours will be defined as ending at 10 am and beginning after 4 pm. You must be an advanced user to use off peak hours. (This kind of use requires that you get building access and room access well in advance of intended off peak use.)
Hourly Fees for Usage of the Keyence BZ-X810 High Content Imaging System
| User Category | UMKC | Academic (non-UMKC) | Commercial |
| Assisted | $15.00 | $20.00 | Inquire |
| Independent | $10.00 | $15.00 | Inquire |
| Time series*/Off peak** | $5.00 | n/a | Inquire |
| Fee-for-Service | Inquire | $75.00 | Inquire |
* Time series extending more than 8 hours ($600 cap for long term time-lapse experiments)
**Off Peak hours will be defined as ending at 10 am and beginning after 4 pm. You must be an advanced user to use off peak hours. (This kind of use requires that you get building access and room access well in advance of intended off peak use.)
New or young investigators who do not yet have funding and are applying for NIH funding may apply for a fee waiver for an initial period of use of the microscope not to exceed 20 hours in order to generate preliminary data for grant applications.
Scheduling
Instrument reservations and/or instrument training sessions can be made on the Confocal and Multiphoton Reservation site (Please remember to reserve both the instrument and a member of the staff if it is your first time using the instrument):
https://umkcconfocal.bookedscheduler.com
Biosafety, IRB, and IACUC approvals.
As part of the user registration process, biosafety concerns will be reviewed with the PI and appropriate IRB, biosafety or IACUC approval will be confirmed. Before any biohazardous samples are viewed on the confocal microscope, appropriate biohazard containment protocols must be in place and approved by the UMKC Biosafety committee.
Radiation
The confocal microscopy core is not approved to handle radioactive materials and cannot accept any such samples.

Tips
For best results when affixing a coverslip to a glass slide use a Toluene free nail polish such as “Wild Shine” to seal cover slips on slides.
Check fixation method for your fluorophores and samples. Some fluorophores and tissues require special fixation considerations.
SpectraViewers for Common Fluorophores:
https://www.thermofisher.com/order/fluorescence-spectraviewer#!/
https://www.chroma.com/spectra-viewer
https://www.perkinelmer.com/lab-products-and-services/spectraviewer
SpectraViewer for Common Fluorescent Proteins: